Ohga Group
Research Topic 1
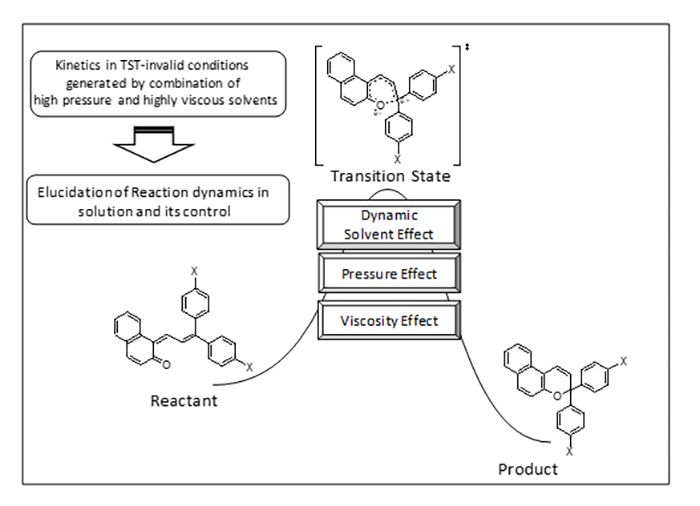
Kinetic Study on Solvent Dynamics in Liquid-Phase Organic Reactions
In combination with viscous liquids, high pressure was effectively used to study dynamic solvent effects on the rate of thermal isomerization of molecules at their electronic ground state. Such studies are expected to yield fundamental knowledge on dynamic details of chemical reactions in solutions. At higher viscosities, if the solvent thermal fluctuations are not fast enough to maintain the thermal equilibrium between the initial and the transition states, the solvent rearrangement in the solvation shell can be rate-determining. Under such conditions, a reactant molecule has to wait for the formation of the solvation sphere that stabilizes the activated complex before it undergoes chemical transformation. In other words, solvent reorganizations and chemical structural changes had to be described by two separate coordinates, i.e., the medium and the chemical coordinate, respectively. We proposed the application of the viscosity effect to the elucidation of dynamic routes of solution reactions. There are two possibilities in solvent reorganizations. The first is that reorganizations take place around the whole reactant molecule without any preference. The second possibility is that solvent molecules are reorganized mainly around a particular part of the reactant. In the first case, any structural modification of the reactant would result in a change in the viscosity dependence. In the second case, only a modification of the moiety around which solvent molecules are reorganized would exert influences on the viscosity effect. We demonstrated that, during the cyclization of chromene derivatives, the substituted ethenyl group changed its position in the solvation sphere while the naphthalenone group stayed in the same position. To the best of our knowledge, this is the first successful attempt to demonstrate the existence of spatial selectivity in the solvent reorganizations in solution reaction.
The dynamic solvent effect of cyclization reaction of chromene derivatives having several polar substituents was also studied in protic and aprotic solvents. Larger dynamic solvent effect in protic solvent might be indicated the hydrogen bonding interaction would be a large microscopic friction.
The asynchornism between the solvent reorganizations and the chemical structural changes and the spatial selectivity in the solvent reorganizations may be considered as two fundamental features of solution reactions.
Publications
- K. Sugita, Y. Goto, M. Ono, K. Yamashita, K. Hayase, T. Takahashi, Y. Ohga, and T. Asano, “A New Application of High-Viscosity Kinetics. An Attempt to Identify a Site of Solvent Reorganizations around a Reactant”, Bull. Chem. Soc. Jpn., 77, 1803-1806 (2004).
- Y. Ohga, Y. Kakitsuba, K. Suzuki, Y. Arakawa, J. Murawaki, Y. Amano, T. Takahashi, and K. Iio, “Photochromic Behaviour of 3,3-Diaryl-3H-naphtho[2,1-b]pyrans Having Polar Substituents on Aryl Groups. Electronic Substituent Effect and Dynamic Solvent Effect on Thermal Fading Process”, Journal of Photocatalysis Science, 3, 41- 47 (2012).
- Y. Shigemitsu and Y. Ohga, “Computational Analysis of Solute–Solvent Coupling Magnitude in the Z/E Isomerization Reaction of Nitroazobenzene and Benzylideneanilines”, J. Solution Chem., 47, 127-139 (2018)
- Y. Shigemitsu and Y. Ohga,”Numerical Analysis of Solute–Solvent Coupling Magnitude in the Thermally Backward Ring Closing Reaction of Spirooxazines”, J. Solution Chem., 49, 902-914 (2020).
- R. Akisaka, Y. Ohga, and M. Abe, “Dynamic Solvent Effects in Radical–radical Coupling Reactions: An Almost Bottleable Localised Singlet Diradical”, Phys. Chem. Chem. Phys., in press. doi: 10.1039/d0cp05235c
Research Topic 2
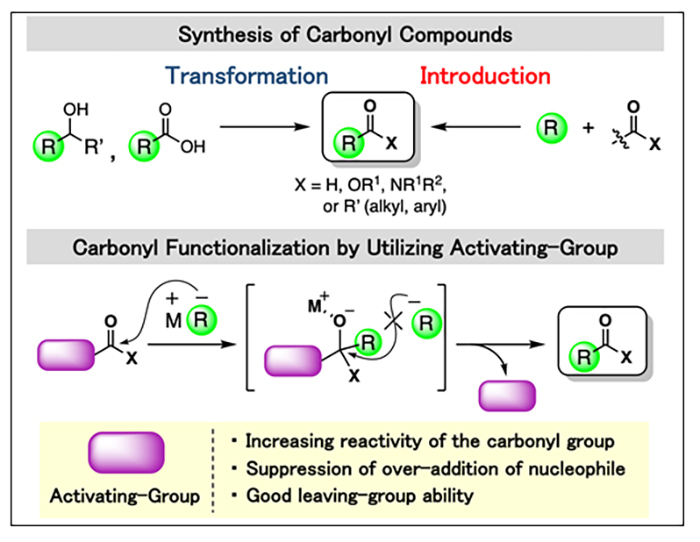
Carbonyl functionalization by utilizing activating-group induced sp2 carbon-heteroatom bond activation
Carbonyl compounds are important organic compounds either as fundamental building blocks in organic synthesis or as valuable end products. They are widely present in pharmaceutical molecules and functional materials. Moreover, carbonyl group can be easily converted to other functional groups. Due to their importance, a number of methods for the synthesis of carbonyl compounds have been developed over the past century. Conventionally, their synthetic methods can be divided into two classes, one involving the transformation to carbonyl compound (such as the oxidation of alcohols and the condensation of carboxylic acids) and the other involving the introduction of a carbonyl group into a carbon skeleton (such as the nucleophilic acyl substitution). Although the transformation reaction is the most commonly used method for preparation of carbonyl compounds, the structure of the carbonyl products depends largely on the substrates. On the other hand, the introduction reaction is a highly flexible synthesis method in which a carbonyl functional group can be introduced into the carbon skeleton and various carbonyl compounds can be synthesized without depending on the substrates. However, this method tends to suffer from drawbacks such as strict reaction conditions or the formation of byproducts by over-addition.
To resolve the synthetic problems of the transformation reaction, we explore for the activating-group applying appropriate reactivity to the carbonyl group and investigate the reactions using these activating-groups.
Publications
- S. Hirao, Y. Sugiyama, M. Iwao, and F. Ishibashi, “Synthetic Approach toward the Telomerase Inhibitor Dictyodendrin B: Synthesis of the Pyrrolo[2,3-c]carbazole Core”, Biosci. Biotech. Bioch., 73, 1764-1772 (2009).
- S. Hirao, Y. Yoshinaga, M. Iwao, and F. Ishibashi, ”A Formal Total Synthesis of the Telomerase Inhibitor Dictyodendrin B”, Tetrahedron Lett., 51, 533-536 (2010).
- S. Hirao, K. Kobiro, J. Sawayama, K. Saigo, N. Nishiwaki, “Ring Construction via Pseudo-intramolecular Hydrazonation Using Bifunctional δ-Keto Nitrile” Tetrahedron Lett., 53, 82-85 (2012)
- H. Asahara, A. Kataoka, S. Hirao, N. Nishiwaki, “Functionalization of a Pyridine Framework through Intramolecular Reissert-Henze Reaction of N-(Carbamoyloxy)pyridinium Salts and Unexpected Insertion of Ethereal Solvents” Eur. J. Org. Chem., 18, 3994-3999 (2015)
- F. Ishibashi, T. Fukuda, S. Zha, A. Hashirano, S. Hirao, and Masatomo Iwao, “Concise synthesis and in vitro anticancer activity of benzo[g][1]benzopyrano[4,3-b]indol-6(13H)-ones (BBPIs), topoisomerase I inhibitors based on the marine alkaloid lamellarin”, Biosci. Biotech. Bioch., in press.




